hankyoreh
Links to other country sites 다른 나라 사이트 링크
Race for vaccine development sparks fierce competition between US and China
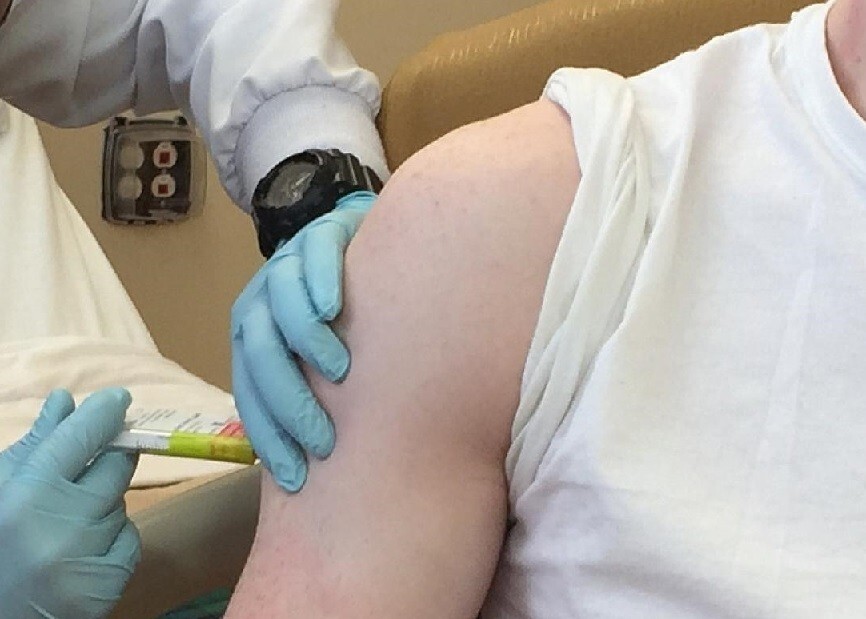
A race for vaccine development is heating up around the world as countries gear up for a long-term battle with the novel coronavirus. Competition between the US and China has been especially fierce. The two countries have been neck and neck as they have begun clinical trials on coronavirus vaccines faster than expected. Two big factors in accelerating the schedule have been the Chinese leadership’s efforts to make up for botching the early response to the virus and strong backing from US President Donald Trump as he eyes re-election. But with the two sides in a race for bragging rights, some are voicing concerns that safety in vaccine testing could end up neglected in the development process.
The US made the first move, with its National Institutes of Health (NIH) announcing in Mar. 16 that it had begun clinical trials on the world’s first vaccine for the novel coronavirus. The very next day, the Academy of Military Medical Sciences (AMMS) under China’s PLA Academy of Military Science also announced that it had begun clinical trials.
The US vaccine was jointly developed by the pharmaceutical company Moderna and the National Institute of Allergy and Infectious Diseases (NIAID). Moderna began a six-week clinical trial process on 45 healthy volunteers aged 18 to 55 at the Kaiser Permanente Washington Health Research Institute in Seattle. Participants are receiving two vaccine injections one month apart -- a phase 1 clinical trial to test the vaccine’s soundness.
Called “mRNA-1273,” the vaccine that Moderna is testing uses viral genetic information. It’s a messenger RNA (mRNA) vaccine that includes genetic information for the protein spikes that protrude from the virus’s surface. The aim is to inject it into the body to encourage cells to create antibodies for the fake spike proteins before any real ones become introduced. The spike proteins are used by the virus as a means of infiltrating human cells.
According to KAIST assistant professor Kim Ho-min of the Institute for Basic Science’s biomolecule and cell structure research group, it would also be possible to create and inject fake spike proteins directly, but the approach of creating them within the body was used because of how much longer that process would take. Moderna originally planned to begin phase 1 of clinical testing in April after completing the structural design and test production within 25 days. But with the encouragement of the Trump administration, it moved its schedule up by nearly a full month.
In China, 108 residents aged 18 to 60 from the Wuhan region of Hubei Province have taken part in clinical trials. Researchers divided them into three groups that each received different quantities of vaccine, after which they were placed in isolation facilities for two weeks for observation. They are to receive regular antibody formation checks over the next six months. According to the researchers’ clinical trial plan, the trials are to continue through the end of 2020.
The Chinese vaccine is a virus recombinant vaccine (adenovirus type 5 vector) developed by AMMS in conjunction with the Tianjin-based bioengineering company CanSino Biologics. It’s based on the technology used for development of an Ebola vaccine. The vaccine makes use of an adenovirus as a gene vector: a gene for producing particular novel coronavirus proteins is inserted into the adenovirus and injected into the human body to induce an immune response. The method reportedly showed a strong immune response and safety in pre-clinical animal trials. Phase 1 clinical trials do not involve verifying a vaccine’s effects through actual infection, but only tests whether antibody formation is induced. According to the New York Times, around 1,000 scientists are taking part in the vaccine research spearheaded by AMMS.
There’s likely to be quite a bit of rivalry between the two countries about the timing of the first vaccine inoculation. On Mar. 19, Zhong Nanshan, an academician at the Chinese Academy of Engineering, said that he expects inoculation in the human body will become possible in September. Then on Mar. 23, Moderna CEO Stephane Bancel said the company plans to put the vaccine on the market next year but make it available in emergency cases this fall.
Countries in Europe, which has recently become a new epicenter of COVID-19, have been racing to develop a vaccine as well. Researchers at Oxford University are planning to complete animal trials on the vaccine between the end of March and early April and to launch clinical trials in April. The US and Germany have been squabbling over the ownership of a vaccine that’s under development at German biotech firm CureVac.
The people who are participating in the clinical trials expressed quite a bit of nervousness, as well as pride about helping develop the world’s first vaccine for COVID-19.
Neal Browning, a Microsoft engineer in Seattle who’s taking part in Moderna’s clinical trials, spoke about his experiences in an interview with online news website Futurism. “If I am healthy and can help take part in trying to reduce the pain, suffering and deaths associated with this pandemic, I would be remiss not to,” Browning said.
Browning was the second member of the cohort to be injected with the experimental vaccine. “There is no dead or even weakened virus in this vaccine, and while it had never been tested on any animal [. . .], I knew and accepted the risks.”
Chao Ren, one of the participants in the clinical trials for the vaccine in Wuhan, China, uploaded a video of her injection on the Douyin video sharing platform (known internationally as Tik Tok). “If my participation makes it possible for people to see each other smiling without wearing a mask, it will be worth it,” Chao said.
Chao said that after hearing that volunteers were needed for the clinical test on Mar. 17, he went in for a checkup the very next day. He reported feeling “sort of proud” and “a little nervous” but added that he trusts the scientists.
But others are worried that intermediary steps are being skipped because of the urgent need to develop a vaccine. For its vaccine, Moderna omitted the animal testing that typically goes before clinical testing. They didn’t start testing the vaccine on rats until the day they started recruiting subjects for the clinical tests.
Necessity of checking for potentially harmful responses in animal studiesAmong those concerned is Jiang Shibo, professor of microbiology at the medical school at Fudan University, in Shanghai. “Regulators must continue to require that vaccine developers check for potentially harmful responses in animal studies. They must also be careful to assess healthy human volunteers [. . .] before enrolling them in safety trials,” Jiang argued in a column that ran recently in science journal Nature.
“China is advancing several COVID-19 vaccines of different types, and has announced plans to have human trials [. . .] in April. My worry is that this could mean a vaccine is administered before its efficacy and safety have been fully evaluated in animal models or clinical trials,” Jiang added.
Jiang also addressed the vaccine based on messenger RNA that has been developed by Moderna. “The mRNA-based platform for delivering vaccines has been shown to be safe in humans, but this COVID-19 vaccine has not,” he said.
“The NIAID argues that the risk of delaying the advancement of vaccines is much higher than the risk of causing illness in healthy volunteers, but I worry that vaccine developers will rush in too hastily if standards are lowered.”
According to Milken Institute, which monitors the development of vaccines and medications, there are currently 43 COVID-19 vaccines being developed around the world, including projects that are still in the pre-clinical stage.
By Kwak No-pil, senior staff writer
Please direct comments or questions to [english@hani.co.kr]

Editorial・opinion
![[Column] Season 2 of special prosecutor probe may be coming to Korea soon [Column] Season 2 of special prosecutor probe may be coming to Korea soon](https://flexible.img.hani.co.kr/flexible/normal/500/300/imgdb/original/2024/0426/3317141030699447.jpg) [Column] Season 2 of special prosecutor probe may be coming to Korea soon
[Column] Season 2 of special prosecutor probe may be coming to Korea soon![[Column] Park Geun-hye déjà vu in Yoon Suk-yeol [Column] Park Geun-hye déjà vu in Yoon Suk-yeol](https://flexible.img.hani.co.kr/flexible/normal/500/300/imgdb/original/2024/0424/651713945113788.jpg) [Column] Park Geun-hye déjà vu in Yoon Suk-yeol
[Column] Park Geun-hye déjà vu in Yoon Suk-yeol- [Editorial] New weight of N. Korea’s nuclear threats makes dialogue all the more urgent
- [Guest essay] The real reason Korea’s new right wants to dub Rhee a founding father
- [Column] ‘Choson’: Is it time we start referring to N. Korea in its own terms?
- [Editorial] Japan’s rewriting of history with Korea has gone too far
- [Column] The president’s questionable capacity for dialogue
- [Column] Are chaebol firms just pizza pies for families to divvy up as they please?
- [Column] Has Korea, too, crossed the Rubicon on China?
- [Correspondent’s column] In Japan’s alliance with US, echoes of its past alliances with UK
Most viewed articles
- 1After election rout, Yoon’s left with 3 choices for dealing with the opposition
- 2AI is catching up with humans at a ‘shocking’ rate
- 3Noting shared ‘values,’ Korea hints at passport-free travel with Japan
- 4Two factors that’ll decide if Korea’s economy keeps on its upward trend
- 5Why Kim Jong-un is scrapping the term ‘Day of the Sun’ and toning down fanfare for predecessors
- 6South Korea officially an aged society just 17 years after becoming aging society
- 7Korea’s 1.3% growth in Q1 signals ‘textbook’ return to growth, says government
- 8Is Japan about to snatch control of Line messenger from Korea’s Naver?
- 91 in 5 unwed Korean women want child-free life, study shows
- 10[Reportage] On US campuses, student risk arrest as they call for divestment from Israel